Quipment overview
Freeze-drying is different from other drying methods. It directly converts the moisture or other organic solvents in the material from a solid state into a gas through sublimation, thereby achieving the purpose of removing moisture or other solvents. Freeze-drying must be frozen at low temperatures and carried out in a vacuum state, so it is also called vacuum freeze-drying. Sublimation refers to the process in which a solvent changes directly from a solid state (such as water, like dry ice) to a gaseous state without passing through the liquid state. The product obtained by freeze-drying is called lyophilisate.
While traditional drying causes the material to shrink and destroy cells, the structure of the sample is not destroyed in freeze-drying because the solid components are supported by the ice in their place. As the ice sublimates, it leaves pores in the dry remaining material. This preserves the integrity of the product's biological and chemical structure and its activity.
Working principle
The working principle of vacuum freeze-drying is to first freeze the wet material below the eutectic point temperature, so that the water becomes solid ice, and then sublimate the ice into water vapor under the appropriate temperature and vacuum degree, and then use the water trap (water vapor condenser) of the vacuum system to condense the water vapor, thereby obtaining the technology of drying products. The drying process is a process of phase change of water and movement of matter. Since this change and movement occurs at low temperature and low pressure, the basic principle of vacuum freeze-drying is the mechanism of heat and mass transfer at low temperature and low pressure.
The basic process of vacuum freeze-drying can be divided into three stages: pre-freezing, sublimation drying and analytical drying.
Technical parameters
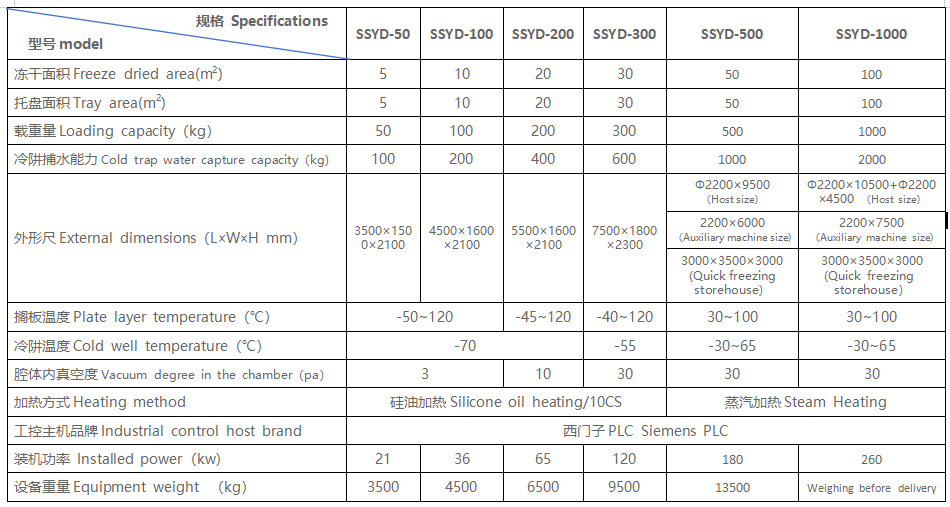
Common terms used in freeze-drying process
1. Eutectic (melting) point temperature.
Materials that need to be freeze-dried must be frozen in advance. The initial materials are mostly solutions, often mixed solutions containing multiple solutes. During the freezing process of materials, as the temperature decreases, the solubility of different solutes in the solution changes. When the concentration is high, the solute is first precipitated from the solution; when the concentration is low, ice crystals are first precipitated from the solution, and the remaining solution forms a eutectic solution, and water and solute reach equilibrium. The temperature at which a eutectic solution begins to freeze is called the eutectic point, which is the highest temperature at which the solution completely freezes and solidifies. For solutions, the freezing solidification point is the starting point of melting, so the eutectic point temperature is also called the eutectic point temperature.
In freeze-drying technology, the eutectic (melting) point temperature is generalized. It does not only refer to the solution, but the temperature at which all materials that need to be freeze-dried reach complete freezing is collectively called the eutectic (melting) point temperature.
There are many methods for measuring the eutectic point temperature, including resistance detection method, differential thermal analyzer scanning method, cryogenic microscope direct observation method, mathematical formula theoretical deduction method, etc. Among them, the resistance detection method is cheap and currently the most widely used. You can make your own measuring device and obtain data directly.
2. Disintegration (collapse) temperature. During the sublimation drying stage of freeze-dried products, as the sublimation time increases, a dried layer and a frozen layer will appear in the product. The interface between these two layers is the sublimation surface. As sublimation proceeds, the sublimation interface continues to move in the direction of reducing the frozen layer, and the thickness of the dry layer gradually increases. The structure of the dried layer product should be loose and porous and maintained in a stable state so that the water vapor sublimated from the frozen layer can pass smoothly so that all products can be dried well. However, when the temperature of some dried products rises to a certain value, they will lose their rigidity and become sticky, causing a collapse phenomenon similar to a collapse, causing the dried products to lose their loose and porous state, blocking the passage for the water vapor in the frozen layer to escape from sublimation, and hindering the continuation of sublimation. As a result, the sublimation rate slows down, and the heat supplied to the frozen layer from the shelf will remain, causing the temperature of the frozen layer product to rise. When the temperature rises above the eutectic point, the product will melt or foam, resulting in freeze-drying failure. The temperature at this time is called the disintegration temperature.
The disintegration temperature of some products is higher than the eutectic point temperature. During sublimation, you only need to control the product temperature to be slightly lower than the eutectic point temperature. The disintegration temperature of some products is lower than the eutectic point temperature. For such products, the disintegration temperature should be controlled. Sublimation at a lower temperature will inevitably extend the freeze-drying time.
The disintegration temperature of a product is difficult to measure and depends on the physical properties of the product itself and the type of protective agent. The disintegration temperature of the mixture depends on the disintegration temperature of each component. Therefore, when selecting a freeze-drying protective agent for a product, materials with a higher disintegration temperature should be selected so that sublimation drying can be performed at a not too low temperature to save freeze-drying energy and time, improve productivity, and reduce costs.
The allowable temperature and disintegration temperature of freeze-dried products are different concepts. The allowable temperature is stipulated based on the quality of the freeze-dried product. When this temperature is exceeded, the freeze-dried product will denature. For example, when vitamin C is freeze-dried, decomposition and destruction will occur when the heating temperature exceeds 40°C. Therefore, its maximum allowable temperature is 40°C.3. Lyophilization timing (sequence)
The freeze-drying sequence refers to the order in which each component of the freeze-drying equipment starts or shuts down at different times during the freeze-drying process. The freeze-drying sequence should be formulated based on the structure and performance of the freeze-drying machine and the process requirements of the freeze-dried product. For example, the pre-freezing of a food freeze-drying machine can be performed outside the drying box, while the pre-freezing of a pharmaceutical freeze-drying machine needs to be performed inside the drying box. The freeze-drying sequence of the two is different. The freeze-drying sequence of a pharmaceutical freeze-dryer is usually as follows.
● Turn on the refrigeration compressor to pre-freeze the product. Existing freeze dryers cannot control the pre-freezing rate. When slow freezing is required, the product is loaded into the freeze-drying box in advance and frozen as the shelf temperature of the freeze dryer decreases; when quick freezing is required, the shelf temperature is lowered first, and then the materials are loaded when the shelf temperature drops to a certain value. Determine the pre-freezing temperature and time according to the nature of the product.
● Turn on the refrigeration compressor to cool down the water catcher. If the freeze dryer and the water trap share a refrigeration unit, a solenoid valve needs to be used to control the refrigeration path; if they use two refrigeration units, the refrigeration compressor can be turned on 30 minutes before the end of pre-freezing.
● Turn on the vacuum pump. When the pre-freezing is completed and the temperature of the water trap reaches -40°C, the vacuum pump is started and the refrigeration of the shelves is stopped.
● Start heating. Generally, heating and controlling the shelf temperature begin when the vacuum degree in the freeze-drying box reaches about 13Pa.
The temperature and vacuum should be controlled throughout the drying process until the end of the drying process. The sequence when shutting down is exactly the opposite to that when starting up. If the freeze-dried product requires capping, the cap should be capped before stopping the machine.
4. Lyophilization curve
The lyophilization curve is usually defined as the plot of shelf temperature versus time. The temperature of the freeze-dried material and the change in pressure in the freeze-drying box over time can also be used as a freeze-drying curve. Each material has a different freeze-drying curve, which is generally determined experimentally and then used to guide freeze-drying production. In a freeze-drying machine with a high degree of automation, as long as the correct freeze-drying curve is input, the machine can automatically perform the freeze-drying process until a qualified product is produced.
Other questions
●The cost of freeze-drying equipment and freeze-dried products is high, freeze-drying takes longer than other drying methods, operating costs are high, and sales prices are expensive. Therefore, freeze-dried products are suitable for drying materials with high added value. However, as people's living standards continue to improve, its application is becoming increasingly widespread.
●There are many types of freeze-dried materials, and their performance parameters are poorly understood. There is a lack of good measurement methods and testing instruments, making it difficult to find the best freeze-drying process. The quality of freeze-dried products is mainly guaranteed by the experience accumulation of producers.
●Freeze-drying theory includes three parts: heat and mass transfer during the pre-freezing and drying process of frozen materials; theory of unsteady temperature field and rarefied gas flow in the freeze-drying machine; theory of solid-vapor phase change of moisture in the material during the pre-freezing process and drying process, and theory of vapor-solid phase change of water vapor in the water trap. At present, only the first part of theoretical research is relatively systematic and in-depth, while the latter two parts of theoretical research are still quite lacking.
Hot Tags: Vacuum freeze dryer Vacuum freeze drying machine Freeze dryer




